10.1 Introduction
As you learned in previous chapters, neurotransmission occurs through the secretion of neurotransmitter from a nerve ending to influence the postsynaptic cell. In this chapter the biological mechanisms of vesicle mediated synaptic transmission will be presented.
Within the last twenty years it has become apparent that all intracellular membrane trafficking is based on the same fundamental mechanisms. The same operations involved in the synthesis and transport of vesicles at the ER and Golgi apparatus in the soma are employed in a modified fashion during neurosecretion. The major difference is that vesicle trafficking during neurosecretion is regulated by Ca2+ influx whereas vesicle trafficking during the synthesis and transport of vesicles is not. Unregulated trafficking is termed constitutive. In both cases, however, trafficking is made up of a series of steps involving budding of vesicles, their docking with other organelles, and fusion with the membranes of these organelles. These processes are believed to be present during vesicle biogenesis when the ER generates vesicles that fuse with the Golgi apparatus, when the endosomes are trafficked to the lysosomes, and when vesicle membrane is recaptured from the nerve ending plasma membrane. There is a budding from their membrane origin, a movement to the destination point, and finally the docking of the vesicles with the target organelle where it attaches to and fuses with the organelle membrane.
This section will cover the life of a neurotransmitter vesicle beginning with its synthesis in the cell soma, transport to the nerve ending, recycling at the nerve ending. Vesicle proteins are eventually returned to the cell soma for reuse in the synthesis of new vesicles.
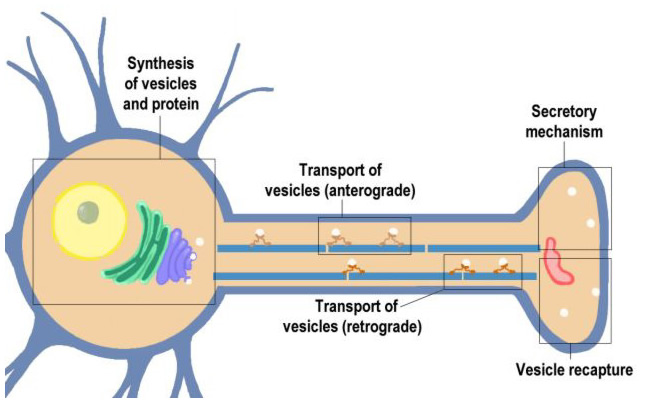
|
Figure 10.1 |
Click on the blocks marked on this Neuron (above) to see details, or choose from the list below.
- Synthesis of Vesicles and Proteins
- Anterograde Transport of Vesicles
- Secretory Mechanism
- Vesicle Recapture
- Retrograde Axoplasmic Transport
10.2 Synthesis of Vesicles and Proteins
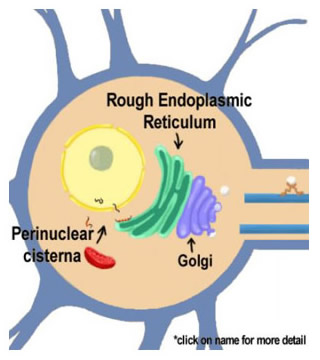
|
Figure 10.2 |
The cycle of neurotransmitter vesicles begins in the ER where the proteins that make up the vesicles are synthesized. Vesicle biosynthesis continues as the proteins migrate through the smooth ER and the Golgi apparatus to eventually emerge to be transported to the nerve ending. The outer layer of the nuclear envelope is contiguous with the endoplasmic reticulum, which is in turn contiguous with the Golgi apparatus.
10.3 Perinuclear Cisternae and Ribosomal Protein Synthesis
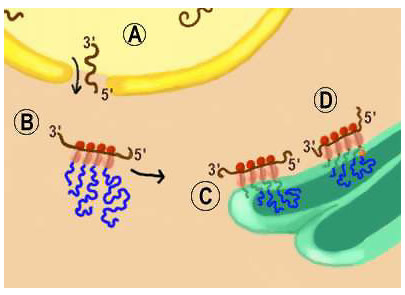
|
Figure 10.3
|
As shown in Figure 10.3, after the mRNA is transcribed from the DNA in the nucleus, it migrates through pores in the nuclear envelope called the perinuclear cisternae (label (A) in Figure 10.3). As the mRNA reaches the cytoplasm it encounters free ribosomes, labeled (B). This is the site of cytoplasmic protein synthesis. Here, triplet nucleotide combinations, called codons, translate the mRNA into protein through a mechanism where ribosomes read off the protein sequence encoded in the mRNA. As ribosomes move along the mRNA template amino acids are added and the protein synthesized. Because most mRNA molecules are longer than a ribosome, many ribosomes can read the codons of a single molecule of RNA.
Click "B" in the graphic at right for animation of cytoplasmic protein synthesis.
Proteins that are destined to be associated with membrane structures, such as neurotransmitter vesicles or the plasmalemma, are synthesized on ribosomes within the endoplasmic reticulum. Recall that the presence of ribosomes defines this part of the endoplasmic reticulum as rough endoplasmic reticulum.
In (C) of Figure 10.3, the polypeptide (protein) being synthesized has no signal peptide. Consequently, this protein will become a non-membrane bound (soluble) protein that resides within lumen of vesicles. While in the lumen, the protein undergoes further processing as it proceeds through the smooth ER, Golgi apparatus, and the secretory vesicle as mature secretory proteins are formed.
Figure 10.4 |
In (D) of Figure 10.3, a signal peptide is a part of the polypeptide being synthesized (this is signified in the figure by the fact that the peptide is anchored in the ribosomal membrane). Consequently, this protein will be an integral membrane protein. As the ribosome moves along the mRNA template, amino acids are added. The signal peptide inserts in the membrane of the endoplasmic reticulum and maintains the protein's association with membrane. This will ensure that the protein will associate with a vesicular structure, such as a neurotransmitter storage vesicle. Shows the protein synthesis from ribosomes in the rough ER to synthesize membrane bound protein.
10.4 Rough Endoplasmic Reticulum
Figures 10.4 illustrates the movement of the secretory vesicles through the rough and the smooth ER. The smooth ER extends from the RER and serves as a site for lipid biosynthesis for the production of endosomes, lysosomes and plasma membrane as well as for the neurotransmitter vesicles. New membrane protein that begins its synthesis in the RER continues in the SER where pieces of the SER bud off to form transport vesicles that shuttle to the Golgi apparatus with their contents.
Figure 10.5 |
As shown in Figure 10.5, in the Golgi apparatus the vesicles fuse to form the outermost of the Golgi apparatus cisternae stacks. Each cisterna migrates in a stepwise fashion through the Golgi apparatus toward the concave surface. During this migration, proteins become more concentrated and undergo various types of biochemical modification to produce mature functional proteins. These modifications include phosphorylation, glycosylation, proteolysis and addition of fatty acids, as well as others. The migration proceeds from the cis face close to the SER to the concave trans face. Upon reaching the concave face the cisternae round up into small vacuoles, then coalesce to form a larger condensing vacuole. The condensing vacuoles then give rise to a number of dense spherical transport vesicles. These vesicles bud off and are transported to the various destinations within the neuron where they become cell membrane, lysosomes, endosomes or neurotransmitter vesicles.
10.6 Anterograde Transport of Vesicles
Figure 10.6 |
Vesicles formed in the cell soma are moved to the site at which they will be used in synaptic transmission. This step in the trafficking of the vesicle is mediated by a process termed fast anterograde axoplasmic transport. As depicted in Figure 10.6, transport is mediated through the interaction of the vesicle with the microtubule. Transport is an energy dependent process in which a so-called motor protein, kinesin, associates with vesicles and moves down the microtubule in a series of attachment-detachment steps. Evidence for the existence of axoplasmic transport comes from a variety of observations, including the movement of radioactive proteins synthesized in the cell soma down the axon to the nerve endings. The speed of transport is 0.5-1.5 cm per hour. Ca2+ is also required for transport. The vinca alkaloid drugs colchicine and vinblastine prevent axoplasmic transport through their disruption of microtubules.
10.7 Secretory Mechanism
As discussed in Chapter 5, neurotransmitter is secreted at the nerve ending through the Ca2+-dependent fusion of neurotransmitter storage vesicle with the plasma membrane with the neurotransmitter being secreted (released) into the synaptic cleft to influence the postsynaptic cell. This process is termed exocytosis. An important concept to emerge is that the same mechanisms that occur in vesicle fusion with membranes in the Golgi during vesicle biosynthesis are also employed at the nerve ending for neurotransmitter release. This is true for vesicle synthesis. Even very simple cells like yeast. This conservation of mechanisms has permitted the use of simple systems to help understand the molecular mechanisms of neurotransmitter release.
Figure 10.7 |
As shown in Figure 10.7, the first event that must occur (with the exception of neuropeptide neurotransmitters) is the filling of vesicles with neurotransmitter through specific neurotransmitter uptake (NT Uptake). This uptake will be covered in subsequent chapters that discuss each of the specific neurotransmitters.
The vesicles remain in reserve until needed for secretion. When needed for secretion, a translocation occurs, which is also referred to as mobilization. The vesicles move to a region of plasma membrane called the active zone. The active zone is the release site and is characterized by the appearance of dense material adjacent to the plasma membrane. The influx of Ca2+ is believed to increase translocation by increasing the Ca2+ dependent phosphorylation of a vesicle binding protein termed synapsin. The theory is that Ca2+ dependent phosphorylation of synapsin frees the vesicles from binding to actin microfilaments. The vesicles then bind to the active zone of the plasma membrane, where they are in position to undergo release of their neurotransmitter.
The association of the vesicle with the plasma membrane is termed docking and serves to prime the vesicle for secretion. The docking is believed to occur through the binding of proteins in the vesicle membrane to proteins in the plasma membrane. Several of these proteins have been discovered because they are targets for clostridia bacterial toxins that block synaptic transanimation. Several of these toxins and the proteins they detect are shown in Table I. The toxins are so toxic that a single molecule can poison a whole nerve terminal. One of the synaptic vesicle proteins is VAMP, and two of the synaptic plasmal membrane proteins are syntaxin and SNAP-25.
| Table I | ||
| Toxin | Synaptic Protein | Location |
| Botulinum toxins A & E | SNAP-25 | Synaptic plasma membrane |
| Botulinum toxin C1 | Syntaxins | Synaptic plasma membrane |
| Botulinum toxin B, D, F & G & tetanus toxin | VAMPs | Synaptic vesicle |
A third plasma membrane protein, n-sec-1, is important because its loose association with the plasma membrane prevents the binding of the neurotransmitter vesicle proteins until n-sec-1 is displaced (the mechanism of n-sec-1 displacement is currently not understood). This and subsequent steps in the secretory process are shown in Figure 10.8. The vesicle and plasma membrane proteins are hypothesized to complex with one another upon the displacement of n-sec-1 to form a "trimeric complex" (SNAP-25, syntaxin and VAMP). This three-member complex has been isolated, intact, from the nerve endings of animals. This association of the proteins initiates fusion. Vesicles at this stage are primed for release.
Figure 10.8 |
The final stage of release, also shown in Figure 10.8, is the fission of the membrane at the point of contact between the vesicle and the plasma membrane. Exocytosis of neurotransmitter into the synaptic cleft occurs when this fission takes place. This step is Ca2+ stimulated, but the mechanism of the Ca2+ trigger is unknown. One hypothesis is that a vesicle protein called synaptotagmin binds Ca2+ to initiate fission. Support for synaptotagmin, as the Ca2+ sensor, is that it possesses two binding sites for Ca2+. Additional evidence comes from studies of mice in which synaptotagmin has been knocked out. In these mice fast Ca2+-triggered synaptic vesicle exocytosis is severely limited. Many aspects of the fusion-fission mechanism remain to be understood, including: what causes the dissociation of n-sec-1 from the complex, how Ca2+ functions in the release process and how all the proteins that are involved in release become reassociated with the proper membrane following release as the vesicle membrane is recycled.
10.8 Vesicle Recapture
Although the source of vesicles for neurotransmitter secretion comes from biosynthesis in the Golgi apparatus at the cell body, evidence indicates that local re-synthesis of synaptic vesicle is an important aspect of neurotransmitter secretion. Figures 10.9 and 10.10 provides two schematic summaries of how vesicles are locally reused. Both utilize the recapture of vesicle membrane from the nerve ending. In one, vesicles bud off the plasma membrane through the formation of pits that migrate directly to become a neurotransmitter vesicle as soon as it can be refilled with neurotransmitter through the neurotransmitter uptake process. This is shown in Figure 10.9. This mechanism is referred to as the "kiss and run" hypothesis. The second mechanism involves the formation of clathrin coated pits to recapture vesicle membrane, and the vesicles cycle through the endosomal compartment in the nerve ending before becoming functional synaptic vesicles. The vesicles then bud off the endosome to form the neurotransmitter vesicle. This is shown in Figure 10.10. It is believed that both mechanisms can exist in the same nerve ending or only one of the two can be present. Both are important in that they recover vesicle protein to permit a plentiful supply for synaptic transmission. This mechanism also prevents the enlargement of the nerve ending that would occur if vesicle membrane were not recaptured. No matter which mechanism is involved, the supply and resupply of vesicles can only keep pace with a high rate of synaptic transmission for a few minutes.
Figure 10.9 |
Figure 10.10 |
|
Representation of two mechanisms proposed to recapture vesicle membrane during neurotransmitter secretion. |
||
10.9 Retrograde Axoplasmic Transport
Figure 10.11 |
Eventually the proteins utilized for synaptic transmission in the nerve ending are targeted and returned to the cell body of the neuron to be recycled to make new protein and vesicles. The proteins are returned to the soma through a retrograde axoplasmic transport that is analogous to anterograde transport but uses a different motor protein, dynein. Transport is mediated by the interaction of dynein with microtubules and proceeds at a rate somewhat slower than that of fast anterograde transport (0.2-1 cm per day). In addition to returning proteins to the soma, retrograde transport serves as a means of communication between the nerve endings and the cell soma. This is a mechanism to transport signaling molecules to regulate the development and maintenance of axonal contacts with postsynaptic cells.
Figure 10.12 |
Figure 10.12 shows a summary of retrograde and anterograde axoplasmic transport. It shows the motor proteins, kinesin and dynein, mediating the movement of vesicles and mitochondria anterogradely and vesicles retrogradely along microtubules. The animation shows the motor proteins as a part of the organelle that is transported. The other possible relationship between the motor protein and microtubules is that the motor proteins are a part of the microtubule and pass the vesicles along the microtubule.
Test Your Knowledge
Which of the following processes dictate the amount of neurotransmitter released from a nerve ending on a short-term, minute-to-minute, basis? (NOTE: there is more than one correct answer.)
Which of the following processes dictate the amount of neurotransmitter released from a nerve ending on a short-term, minute-to-minute, basis? (NOTE: there is more than one correct answer.)
Which of the following processes dictate the amount of neurotransmitter released from a nerve ending on a short-term, minute-to-minute, basis? (NOTE: there is more than one correct answer.)
Which of the following processes dictate the amount of neurotransmitter released from a nerve ending on a short-term, minute-to-minute, basis? (NOTE: there is more than one correct answer.)
Which of the following processes dictate the amount of neurotransmitter released from a nerve ending on a short-term, minute-to-minute, basis? (NOTE: there is more than one correct answer.)
Which of the following processes dictate the amount of neurotransmitter released from a nerve ending on a short-term, minute-to-minute, basis? (NOTE: there is more than one correct answer.)
|






