
Lab Research
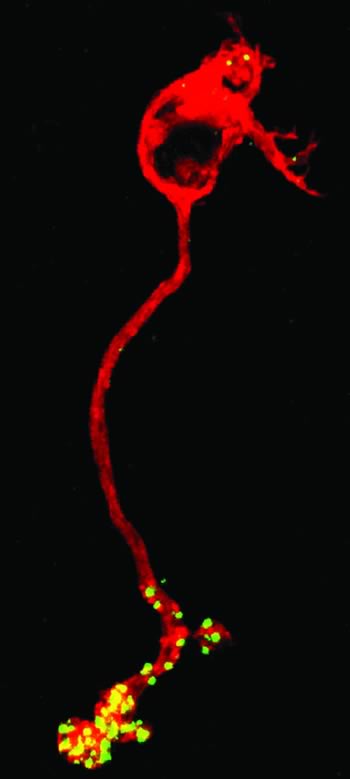 Communication between neurons via chemical synaptic transmission is a fundamental and highly-conserved feature of nervous system function. We seek to understand chemical neurotransmission at the level of synaptic vesicle dynamics. To this end, we study the glutamatergic synapses of photoreceptors and bipolar cells of the vertebrate retina. There are several reasons for our interest in these neurons. First, the synaptic endings of these neurons are unusually large and accessible to biophysical examination. Thus, they allow us to perform detailed analyses of the mechanisms of exocytosis and endocytosis that cannot be readily performed at many other central synapses. Secondly, photoreceptors and bipolar cells comprise the major throughput pathway for visual information within the vertebrate retina. To more fully appreciate how visual information is processed as it traverses the retina, we are examining the factors that regulate transmitter release and synaptic vesicle dynamics at these key synapses. Thirdly, there are subtle molecular differences between the ribbon-style synapses formed by photoreceptors and bipolar cells and those of conventional synapses. We exploit these differences to study the function of select synaptic proteins. To achieve our goals, biophysical approaches such as single cell patch-clamp electrophysiology, time-resolved membrane capacitance measurements and fluorescence measurements of intracellular calcium are often used in combination with computational and molecular approaches.
Communication between neurons via chemical synaptic transmission is a fundamental and highly-conserved feature of nervous system function. We seek to understand chemical neurotransmission at the level of synaptic vesicle dynamics. To this end, we study the glutamatergic synapses of photoreceptors and bipolar cells of the vertebrate retina. There are several reasons for our interest in these neurons. First, the synaptic endings of these neurons are unusually large and accessible to biophysical examination. Thus, they allow us to perform detailed analyses of the mechanisms of exocytosis and endocytosis that cannot be readily performed at many other central synapses. Secondly, photoreceptors and bipolar cells comprise the major throughput pathway for visual information within the vertebrate retina. To more fully appreciate how visual information is processed as it traverses the retina, we are examining the factors that regulate transmitter release and synaptic vesicle dynamics at these key synapses. Thirdly, there are subtle molecular differences between the ribbon-style synapses formed by photoreceptors and bipolar cells and those of conventional synapses. We exploit these differences to study the function of select synaptic proteins. To achieve our goals, biophysical approaches such as single cell patch-clamp electrophysiology, time-resolved membrane capacitance measurements and fluorescence measurements of intracellular calcium are often used in combination with computational and molecular approaches.
Projects
The calcium sensor for graded exocytosis
Do photoreceptors utilize a novel calcium sensor and if so, why? Our previous data suggest that the calcium sensor for release in the rod is highly atypical in that no more than three calcium binding sites need to be occupied in order for release to occur, rather than the expected five. However, an intriguing alternate possibility is that the rod might use a conventional neuronal calcium sensor, but that release occurs from partially-bound states of the sensor, rather than being restricted to the fully-bound state, as is commonly assumed. An attractive feature of this hypothesis is that the relationship between release and calcium will be shallow at low calcium values and grow steeper with increasing calcium. Thus, it is the presynaptic calcium signal that determines whether release varies linearly or supralinearly with calcium. We are exploring the implications of this model and others with respect to calcium-dependent synaptic vesicle dynamics using both physiological and computational approaches. These studies will help us identify critical features of the calcium sensor at ribbon synapses that shape retinal signal processing.
 Synaptic vesicle dynamics
Synaptic vesicle dynamics
Several kinetically discrete components of exocytosis have been characterized at ribbon synapses. We are interested in how these components relate to physical vesicle pools, their mobilization, location, and the regulation of their recruitment. In particular, we are interested in learning the contributions of these different components to phasic release, sustained release and light adaptation. Furthermore, we are interested in how the interchange between these vesicle pools is regulated, as the regulation of such local synaptic vesicle dynamics is a potentially powerful and rapid means by which synaptic output can be regulated.
Molecular mechanisms of exocytosis at ribbon-style synapses
To better understand the molecular mechanisms underlying exocytosis and its regulation, we developed the mouse rod bipolar cell as a model system. We are now using this well-defined mammalian system to ascertain the roles of specific synaptic proteins in release. We are currently looking at the role of proteins that have the potential to be “gain-control” proteins that regulate synaptic output. One such putative gain-control protein has been strongly implicated in human epilepsy, although its function is largely unknown. We are actively working to discover its mechanism of action.
Computational models of exocytosis
In the course of determining the basic features of synaptic vesicle dynamics and calcium-regulated vesicle fusion in each of our model systems, we have gathered much of the data needed to construct computational models of these processes. Additional experiments will provide the rest of the required information, thereby minimizing the number of free parameters in the model. These models will then be invoked to help interpret results of manipulations that alter synaptic protein function and/or synaptic strength and determine the most likely process in the secretory pathway that underlies this change. In addition, while most of the physiological experiments are performed under conditions designed to give measurable results, this is not always the range wherein a neuron functions under physiological conditions. The models will also be used to help predict outcomes under different physiological conditions. Since a computational model is only useful if it can generate testable predictions, where possible, the model predictions will be experimentally tested.
