Sensitivity and reactivity to noxious stimuli are essential to the well-being and survival of an organism. Without these attributes, the organism has no means to prevent or minimize tissue injury. Given the importance of pain sensation, it is not surprising that the central and peripheral neuronal pathways that subserve the transmission of nociceptive information and the pathway that transmits and modulates this somatosensory transmission are essential to understanding this somatosensory modality. Pain travels through “redundant” pathways, ensuring to inform the subject to "Get out of this situation immediately."
7.1 Pain Pathways
The ascending pathways that mediate pain consist of three different tracts: the neospinothalamic tract, the paleospinothalamic tract and the archispinothalamic tract. The first-order neurons are located in the dorsal root ganglion (DRG) for all three pathways. Each pain tract originates in different spinal cord regions and ascends to terminate in different areas in the CNS.
Neospinothalamic Tract
The neospinothalamic tract has few synapses and constitutes the classical lateral spinothalamic tract (LST) (Figure 7.1). The first-order nociceptive neurons (in the DRG) make synaptic connections in Rexed layer I neurons (marginal zone). Axons from layer I neurons decussate in the anterior white commissure, at approximately the same level they enter the cord, and ascend in the contralateral anterolateral quadrant. Most of the pain fibers from the lower extremity and the body below the neck terminate in the ventroposterolateral (VPL) nucleus and ventroposteroinferior (VPI) nucleus of the thalamus, which serves as a relay station that sends the signals to the primary cortex. The VPL is thought to mainly be concerned with discriminatory functions. The VPL sends axons to the primary somatosensory cortex (SCI).
Figure 7.1 |
The first-order nociceptive neurons from the head, face and intraoral structures have somata in the trigeminal ganglion (Figure 7.2). Trigeminal fibers enter the pons, descend to the medulla and make synaptic connections in the spinal trigeminal nucleus, cross the midline and ascend as trigeminothalamic tract (or trigeminal lemniscus, Figure 7.2). The A delta fibers terminate in the ventroposteromedial (VPM) thalamus, and the C fibers terminate in the parafasciculus (PF) and centromedian (CM) thalamus (PF-CM complex). The PF-CM complex is located within the intralaminar thalamus and are known also as intralaminar (IL) nuclei. All of the neospinothalamic fibers terminating in VPL and VPM are somatotopically oriented and from there send axons that synapse on the primary somatosensory cortex (SC I - Brodman areas 1 & 2). This pathway is responsible for the immediate awareness of a painful sensation and for awareness of the exact location of the painful stimulus.
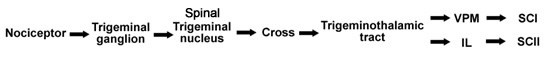
Figure 7.2 |
Paleospinothalamic Pathway
The paleospinothalamic tract is phylogenetically old. The majority of the first-order nociceptive neurons make synaptic connections in Rexed layer II (substantia gelatinosa) and the second-order neurons make synaptic connections in laminae IV-VIII. The second-order neurons also receive input from mechanoreceptors and thermoreceptors. The nerve cells that furnish the paleospinothalamic tract are multireceptive or wide dynamic range nociceptors. Most of their axons cross and ascend in the spinal cord primarily in the anterior region and thus called the anterior spinal thalamic tract (AST). These fibers contain several tracts. Each of them makes a synaptic connection in different locations: 1) in the mesencephalic reticular formation (MFR) and in the periaqueductal gray (PAG), and they are also called spinoreticular tract; 2) in the tectum, and these fibers are known as the spinotectal or spinomedullary tract; 3) in the PF-CM complex (IL) and they are known as the spinothalamic tract (Figure 7.3). The above three fiber tracts are known also as the paleospinothalamic tract. The innervation of these three tracts is bilateral because some of the ascending fibers do not cross to the opposite side of the cord. From the PF and CM complex, these fibers synapse bilaterally in the somatosensory cortex (SC II-Brodman area 3). The paleospinothalamic pathway also activates brain stem nuclei which are the origin of descending pain suppression pathway regulating noxious input at the spinal cord level (see next chapter).
Figure 7.3 |
The multisynaptic tracts which course via the reticular formation also project to the PF-CM (IL) complex. There are extensive connections between the IL and the limbic areas such as the cingulate gyrus and the insular cortex, which is thought to be involved in processing the emotional components of pain. That is to say, the insular cortex integrates the sensory input with the cortical cognitive components to elicit the response to the sensation. The limbic structures, in turn, project to the hypothalamus and initiate visceral responses to pain. The intralaminar nuclei also projects to the frontal cortex, which in turn projects to the limbic structures where the emotional response to pain is mediated.
Archispinothalamic Pathway
The archispinothalamic tract is a multisynaptic diffuse tract or pathway and is phylogenetically the oldest tract that carries noxious information. The first-order nociceptive neurons make synaptic connections in Rexed layer II (substantia gelatinosa) and ascend to laminae IV to VII. From lamina IV to VII, fibers ascend and descend in the spinal cord via the multisynaptic propriospinal pathway (Figure 7.4) surrounding the grey matter to synapse with cells in the MRF-PAG area. Further multisynaptic diffuse pathways ascend to the intralaminar (IL) areas of the thalamus (i.e., PF-CM complex) and also send collaterals to the hypothalamus and to the limbic system nuclei. These fibers mediate visceral, emotional and autonomic reactions to pain.
Figure 7.4 |
Figure 7.5 summarizes the three major spinal thalamic pathways. Information about bodily events is conveyed by primary sensory fibers to higher brain centers through the dorsal column medial lemniscal pathways. This route is considered a "touch pathway," separate from the spinal thalamic pathways. However, recent reports indicate that the dorsal column can also carry noxious information from the viscera and widespread skin regions.
Figure 7.5 |
Somatic Pain
Somatic pain can be classified as either: 1) cutaneous, superficial or peripheral pain and 2) deep pain.
- Cutaneous, Superficial or Peripheral Pain. Pain that arises from the skin and muscles or peripheral nerves themselves. In general, this pain has two components, the initial response (a) followed by later response (b). These signals are transmitted via different pathway.
- Pricking pain reaches the CNS via neospinothalamic tract (i.e., LST) to the VPL (or VPM) and to the SCI.
- Burning and soreness pain resulting from tissue damage reaches the CNS via the paleospinothalamic tract (AST) and archispinothalamic tract to brain stem nuclei and to PF-CM complex, etc.
- Deep pain. This pain arises from joint receptors tendons and fascia (i.e., deep structures). The quality of deep pain is dull, aching or burning. Deep pain is accompanied by a definite autonomic response associated with sweating and nausea, changes in blood pressure and heart rate. Somatic deep pain reaches the CNS mainly via the paleospinothalamic (Figure 7.3) and archispinothalamic tract (Figure 7.4).
Reaction to Somatic Pain. Sudden, unexpected damage to the skin is followed by three responses:
- Startle response. This is a complex psychosomatic response to a sudden unexpected stimulus which includes: A flexion reflex, postural readjustment and orientation of the head and eyes to examine the damaged area.
- Autonomic response. This response includes: NE and E release, ACTH and/or cortisol release, and vasoconstriction and piloerection.
- Behavioral response. This response includes: Vocalization, rubbing designed to diminish pain, learning to respond to sudden pain and psychosomatic pain.
Visceral Pain
In the visceral organs, nociceptors respond to mechanical stimulation such as pressure, tissue damage, and chemical stimulation.
Most noxious information carried by visceral afferents does not give rise to conscious sensation. Visceral pain is diffuse, less precisely graded and typically accompanied by slowing of the heart, lowered blood pressure, cold sweats and nausea. It conveys also hunger, thirst, electrolyte balance, irregulation in the respiratory and circulatory systems. Many of these signals reach the CNS bilaterally (Figure 7.6) by the following three channels:
In the visceral organs, free nerve endings are scattered, and any stimulus that excites these nerve endings causes visceral pain (Figure 7.6). Such stimuli include spasm of the smooth muscle in a hollow viscus, or distention or stretching of the ligament, such as a stone blocking the ureter or the gall ducts. Stretching of the tissues such as intestinal obstruction can also provoke visceral pain. Visceral pain is also caused by chemical means as a result of gastrointestinal lesions, and tumors as well as thrombosis of an artery. In many cases, visceral pain is not localized to the site of its cause, rather in a distant site.
Figure 7.6 |
Thalamic Pain
Stroke or occlusion in the thalamogeniculate artery (a branch of the posterior cerebral artery), which supplies the lateroposterior half of the thalamus, can result in a thalamic lesion, which is often accompanied by neurologic conditions several months after the initial event. The condition is associated with a devastating intracranial pain in the contralateral side of the thalamic lesion and sensory loss. In some cases, severe facial pain is experienced without any sensory loss. The pain resulting from an intracranial lesion is also termed "central pain."
Lesions in the spinothalamic tract and its targets of termination as well as local manifestations of diencephalic lesions are usually complex. They can induce alteration of sensory, motor and endocrine components because of the functional diversity of the thalamus. Subjects with this syndrome experience spontaneous aching and burning pain in body regions where sensory stimuli normally do not lead to pain. Because the brain and the spinal cord do not contain nociceptors, the pathological process presumably directly stimulates nociceptive pathways, or it prevents the activation of the pain suppression pathways. This condition is known also as thalamic pain syndrome or Dejerive-Roussy syndrome.
Neuropathic Pain
Neuropathic pain is a sharp, shooting and devastating pain. It is a persistent pain that arises from functional changes occurring in the CNS secondary to peripheral nerve injury. Once the nerve is damaged, the damaged nerve elicits sustained activation of nociceptors and/or nociceptive afferents. The neuropathic pain is due to an abnormal activation of the nociceptive system without specifically stimulating the nociceptors. Neuroplastic changes occurring in the CNS secondary to the afferent barrage are believed to culminate in CNS neuronal hyperexcitability. Many scientists suggest that “sensitization” of the nervous system following injury is a factor in neuropathic pain. Neuropathic pain can usually be controlled by anti-inflammatory drugs and opioids. In some cases, such as in diabetics, AIDS, cancer, etc., no treatment or relief is available to neuropathic pain. Neuropathic pain should not be confused with neurogenic pain, a term used to describe pain resulting from injury to a peripheral nerve but without necessarily implying any neuropathy.
Psychosomatic Pain
Psychic reaction to pain includes all the well-known responses to pain such as anguish, anxiety, crying, depression, nausea and excess muscular excitability through the body. These reactions vary tremendously from one person to another following a comparable degree of pain stimuli. The sensation of pain can be influenced by emotions, past experiences and suggestions. The same stimulus can elicit different responses in different subjects under the same conditions.
Recently, Positron Emission Tomography (PET) has been used to study pain pathways and psychosomatic pain centers. For example, volunteers had their hands dipped in hot water (50° C) while they were conscious. They then dipped their hand again in hot water (50° C) after a post-hypnotic suggestion that the pain would be either more or less unpleasant than the first time. The PET scans of their brains showed that activity in the anterior cingulate cortex changed in accordance with how unpleasant they expected the pain to be. However, the intensity in the primary somatosensory cortex remained constant (i.e., the emotional component of pain is independent of its sensation).
Referred pain is a painful sensation at a site other than the injured one. The pain is not localized to the site of its cause (visceral organ) but instead is localized to a distant site. One possible exception is that the axons carry pain information from the viscera enter into the spinal cord by the same route as the cutaneous pain sensation axons. Within the spinal cord there is a convergence of the information on the same nocineurons. This convergence gives rise to the phenomenon of referred pain. For example, pain associated with angina pectoris, or myocardial infarction is referred to the left chest, left shoulder, and upper left arm (Figure 7.7). Pain resulting from distention of the colon is referred to the periumbilical area (Figures 7.8).
The following are some hypothesis to explain referred pain
- Common dermatome hypothesis. When pain is referred, it is usually to a structure that developed from the same embryonic segment or dermatome as the structure in which the pain originates. Radiating pain down the left arm is the result of a myocardial infarction (Figure 7.7), or pain originating from the shoulder (dermatomes 3-5).
- Convergence and facilitation theories (Figure 7.9). Inputs from visceral and skin receptors converge on the same spinal cord neuron (i.e., viscerosomatic neurons). Therefore, visceral pain is referred to skin area because the nociceptors' terminals from the viscera terminate in the spinal cord on the same neurons that receive input from the skin.
Figure 7.9
Convergence in referred pain is carried via the paleospinothalamic tract. - Facilitation or irritable focus. Pain impulses from the viscera alone are unable to pass directly from spinal cord neurons to the brain, but create an "irritable focus". When visceral and skin impulses arrive together, the information transmitted to higher centers and the brain interprets the pain as being from the skin (Figure 7.10).
Figure 7.10
Convergence of referred pain. - Learned phenomenon. Visceral information arrives in the CNS. However, the brain interprets that the impulses originate from the site of a previous surgical operation, trauma or localized pathologic process.
Phantom (illusory) Pain
Phantom or illusory pain is the experience of pain without any signals from nociceptors. It occurs in a subject with previous injuries such as amputation in which the dorsal roots are literally absent from the cord. Even though no sensory signals can enter the cord, the subject often feels extreme pain in the denervated parts of the body. For example, an amputee will often apparently feel pain in a part of his body that has been removed. The phenomenon of phantom limb pain is a common experience after a limb has been amputated or its sensory roots have been destroyed in which the pain is felt in a part of the body that no longer exists. Pain from an amputated arm is referred to the viscera as a result of disruption to the “balance” between different peripheral inputs to the dorsal horn. A complete break of the spinal cord also often leads to a phantom body pain below the level of the break. The source of phantom pain is complex and not well understood. It has been suggested that there may be abnormal discharges 1) from the remaining cut ends of nerves which grow into nodules called neuromas, 2) from overactive spinal neurons, 3) from abnormal flow of signals through the somatosensory cortex, or 4) from burst-firing neurons in the thalamus.
Acute pain arises from activation of nociceptors for a limited time and is not associated with significant tissue damage (e.g., a pin prick).
7.4 Chronic Pain
Chronic pain is prolonged pain lasting for months or longer that arises from tissue injury, inflammation, nerve damage, tumor growth, lesion or occlusion of blood vessels. Chronic or inflammatory pain can sensitize (see "Sensitization" below) the nervous system, evoking chemical, functional, and even structural changes that serve to “prime the pain-processing pump”. Chronic pain, such as lower back pain, rheumatoid and osteoarthritis, and headache (see "Headaches" below) may result from constant inflammatory activity which activates G proteins. In some cases, the pain persists long after the injury heals, but there is no treatment that will eliminate the pain.
Sensitization
One possible explanation for chronic pain is a phenomenon called sensitization. Following continuation and prolong noxious stimulation, nearby silent nociceptive neurons that previously were unresponsive to stimulation, now become responsive. In addition, some of the chemicals produced and released at the injured site also alter the physiological properties of nociceptors. The nociceptors begin to initiate pain signals spontaneously, which cause chronic pain. In addition, weak stimuli, such as a light touch that previously had no effect on these nociceptors, will further activate the nociceptors which result in severe pain signals. This phenomenon is referred to as “peripheral sensitization.” The outcome of peripheral sensitization results in a greater and more persistent barrage of nerve impulses firing in the CNS. The persistent barrage of nerve impulses results in long-term changes in nerve cell activity at the level of the spinal cord and higher centers in the brain. This phenomena is referred to as “central sensitization”. It appears that peripheral and central sensitization persists after the injury apparently has healed. The sensitization of nociceptive neurons after injury results from the release of different chemicals from the damaged area. It is known that substance P and calcitonin gene-related peptides are released from peripheral nerve ending which stimulate most cells to release algesic substances which further potentiates the pain from the injury. In contrast, central sensitization resulting from severe and persistent injury which cause prolonged release of glutamate on nociceptive dorsal horn cells, this constant glutamate release via G protein dependent phosphorylation cascades results in opening of postsynaptic ion channels gated by the NMDA receptors. This phenomenon is also termed "wind up." This activation produces hyperexcitability of the dorsal horn cells and causes "central sensitization." Pain experts now agree that treating chronic pain early and aggressively yields the best results and prevents patients from developing physical and psychological conditions that could worsen the pain.
Fibromyalgia
Fibromyalgia is characterized by widespread chronic pain throughout the body, including fatigue, anxiety and depression. It is now believed that it has a genetic component which tends to run in families.
Headaches
A headache is a poorly understood type of pain that can be either acute or chronic. There are about 300 different types and causes of headaches. The following are some categories of disorders associated with headaches:
- Intracranial structural disease
- Infectious disease
- Cerebrovascular ischemia
- Cerebral vein thrombosis
- Metabolic disease
- Toxic exposures
- Medications
- Extracranial pressure disorders
- Sinusitis
- Vasculitis and collagen vascular disease
- Hemorrhage (parenchymal and subarachnoid)
- Trauma
- Withdrawal syndromes
- Severe hypertension
- Dental, cranial vault, TMJ, and myofascial disorders
- Cervical spine and occipitocervical junction disorders
7.5 Summary
Because of the importance of warning signals of dangerous circumstances, several nociception pathways are involved to transmitting these signals and some of them are redundant.
The neospinothalamic tract conducts fast pain (via A delta fibers) and provides information of the exact location of the noxious stimulus, and the multisynaptic paleospinothalamic and archispinothalamic tracts conduct slow pain (via C fibers), a pain which is poorly localized in nature. (Figure 7.5)
Pain activates many brain areas, which link sensation, perception, emotion, memory and motor reaction. Therefore, many pain clinics target their treatments to block the perception of pain using psychosomatic means of treatments such as biofeedback, hypnosis, physical therapy, electrical stimulation, and acupuncture-multimodal treatment.
- Question 1
- A
- B
- C
- D
- E
Mr. John Thomas experiences visceral pain around the upper left lung. All of the following carry this nociceptive information EXCEPT the:
A. somatic nerves
B. paleospinothalamic tract
C. sympathetic nerves
D. neospinothalamic tract
E. archispinothalamic tract
Mr. John Thomas experiences visceral pain around the upper left lung. All of the following carry this nociceptive information EXCEPT the:
A. somatic nerves This answer is INCORRECT.
B. paleospinothalamic tract
C. sympathetic nerves
D. neospinothalamic tract
E. archispinothalamic tract
Mr. John Thomas experiences visceral pain around the upper left lung. All of the following carry this nociceptive information EXCEPT the:
A. somatic nerves
B. paleospinothalamic tract This answer is INCORRECT.
C. sympathetic nerves
D. neospinothalamic tract
E. archispinothalamic tract
Mr. John Thomas experiences visceral pain around the upper left lung. All of the following carry this nociceptive information EXCEPT the:
A. somatic nerves
B. paleospinothalamic tract
C. sympathetic nerves This answer is INCORRECT.
D. neospinothalamic tract
E. archispinothalamic tract
Mr. John Thomas experiences visceral pain around the upper left lung. All of the following carry this nociceptive information EXCEPT the:
A. somatic nerves
B. paleospinothalamic tract
C. sympathetic nerves
D. neospinothalamic tract This answer is CORRECT!
The neospinothalamic tract carries nociceptive information from the skin only via A delta fibers, while visceral pain is carried via C fibers.
E. archispinothalamic tract
Mr. John Thomas experiences visceral pain around the upper left lung. All of the following carry this nociceptive information EXCEPT the:
A. somatic nerves
B. paleospinothalamic tract
C. sympathetic nerves
D. neospinothalamic tract
E. archispinothalamic tract This answer is INCORRECT.
- Question 2
- A
- B
- C
- D
- E
A surgeon attempting to treat chronic pain from the pelvic region will suggest to make a lesion in the:
A. somatosensory cortex
B. ventroposterior medial thalamus
C. anterior white commissure
D. dorsal column
E. anterior lateral funiculus
A surgeon attempting to treat chronic pain from the pelvic region will suggest to make a lesion in the:
A. somatosensory cortex This answer is INCORRECT.
B. ventroposterior medial thalamus
C. anterior white commissure
D. dorsal column
E. anterior lateral funiculus
A surgeon attempting to treat chronic pain from the pelvic region will suggest to make a lesion in the:
A. somatosensory cortex
B. ventroposterior medial thalamus This answer is INCORRECT.
C. anterior white commissure
D. dorsal column
E. anterior lateral funiculus
A surgeon attempting to treat chronic pain from the pelvic region will suggest to make a lesion in the:
A. somatosensory cortex
B. ventroposterior medial thalamus
C. anterior white commissure This answer is INCORRECT.
D. dorsal column
E. anterior lateral funiculus
A surgeon attempting to treat chronic pain from the pelvic region will suggest to make a lesion in the:
A. somatosensory cortex
B. ventroposterior medial thalamus
C. anterior white commissure
D. dorsal column This answer is INCORRECT.
E. anterior lateral funiculus
A surgeon attempting to treat chronic pain from the pelvic region will suggest to make a lesion in the:
A. somatosensory cortex
B. ventroposterior medial thalamus
C. anterior white commissure
D. dorsal column
E. anterior lateral funiculus This answer is CORRECT!
Anterior lateral corodotomy interrupt the spinothalamic tract carrying the pain sensation.
- Question 3
- A
- B
- C
- D
- E
In Brown-Sequard syndrome:
A. Tactile and pain sensation are lost contralaterally at different levels below the lesion.
B. Thermal sensation is lost in the ipsilateral side above the lesion.
C. Kinesthetic and tactile senses are lost ipsilaterally below the lesion.
D. The withdrawal reflex is lost.
E. Atrophy is developed in the muscles below the lesion.
In Brown-Sequard syndrome:
A. Tactile and pain sensation are lost contralaterally at different levels below the lesion. This answer is INCORRECT.
B. Thermal sensation is lost in the ipsilateral side above the lesion.
C. Kinesthetic and tactile senses are lost ipsilaterally below the lesion.
D. The withdrawal reflex is lost.
E. Atrophy is developed in the muscles below the lesion.
In Brown-Sequard syndrome:
A. Tactile and pain sensation are lost contralaterally at different levels below the lesion.
B. Thermal sensation is lost in the ipsilateral side above the lesion. This answer is INCORRECT.
C. Kinesthetic and tactile senses are lost ipsilaterally below the lesion.
D. The withdrawal reflex is lost.
E. Atrophy is developed in the muscles below the lesion.
In Brown-Sequard syndrome:
A. Tactile and pain sensation are lost contralaterally at different levels below the lesion.
B. Thermal sensation is lost in the ipsilateral side above the lesion.
C. Kinesthetic and tactile senses are lost ipsilaterally below the lesion. This answer is CORRECT!
Thermal and pain sensation are lost contralaterally below the lesion while kinesthetic and tactile senses remain on the ipsilateral side.
D. The withdrawal reflex is lost.
E. Atrophy is developed in the muscles below the lesion.
In Brown-Sequard syndrome:
A. Tactile and pain sensation are lost contralaterally at different levels below the lesion.
B. Thermal sensation is lost in the ipsilateral side above the lesion.
C. Kinesthetic and tactile senses are lost ipsilaterally below the lesion.
D. The withdrawal reflex is lost. This answer is INCORRECT.
E. Atrophy is developed in the muscles below the lesion.
In Brown-Sequard syndrome:
A. Tactile and pain sensation are lost contralaterally at different levels below the lesion.
B. Thermal sensation is lost in the ipsilateral side above the lesion.
C. Kinesthetic and tactile senses are lost ipsilaterally below the lesion.
D. The withdrawal reflex is lost.
E. Atrophy is developed in the muscles below the lesion. This answer is INCORRECT.
- Question 4
- A
- B
- C
- D
- E
Sharp localized pain is transmitted by:
A. archispinothalamic tract
B. Paleospinothalamic tract
C. Neospinothalamic tract
D. Sympathetic nerves
E. Parasympathetic nerves
Sharp localized pain is transmitted by:
A. archispinothalamic tract This answer is INCORRECT.
B. Paleospinothalamic tract
C. Neospinothalamic tract
D. Sympathetic nerves
E. Parasympathetic nerves
Sharp localized pain is transmitted by:
A. archispinothalamic tract
B. Paleospinothalamic tract This answer is INCORRECT.
C. Neospinothalamic tract
D. Sympathetic nerves
E. Parasympathetic nerves
Sharp localized pain is transmitted by:
A. archispinothalamic tract
B. Paleospinothalamic tract
C. Neospinothalamic tract This answer is CORRECT!
Sharp pain is carried by the neospinothalamic tract.
D. Sympathetic nerves
E. Parasympathetic nerves
Sharp localized pain is transmitted by:
A. archispinothalamic tract
B. Paleospinothalamic tract
C. Neospinothalamic tract
D. Sympathetic nerves This answer is INCORRECT.
E. Parasympathetic nerves
Sharp localized pain is transmitted by:
A. archispinothalamic tract
B. Paleospinothalamic tract
C. Neospinothalamic tract
D. Sympathetic nerves
E. Parasympathetic nerves This answer is INCORRECT.
- Question 5
- A
- B
- C
- D
- E
Select the best answer: Pain impulses arising within the abdominal and thoracic cavities may reach the CNS by:
A. somatic nerves innervating
B. sympathetic nerves
C. parasympathetic nerves
D. none of the above
E. all of the above
Select the best answer: Pain impulses arising within the abdominal and thoracic cavities may reach the CNS by:
A. somatic nerves innervating This answer is PARTIALLY correct.
B. sympathetic nerves
C. parasympathetic nerves
D. none of the above
E. all of the above
Select the best answer: Pain impulses arising within the abdominal and thoracic cavities may reach the CNS by:
A. somatic nerves innervating
B. sympathetic nerves This answer is PARTIALLY correct.
C. parasympathetic nerves
D. none of the above
E. all of the above
Select the best answer: Pain impulses arising within the abdominal and thoracic cavities may reach the CNS by:
A. somatic nerves innervating
B. sympathetic nerves
C. parasympathetic nerves This answer is PARTIALLY correct.
D. none of the above
E. all of the above
Select the best answer: Pain impulses arising within the abdominal and thoracic cavities may reach the CNS by:
A. somatic nerves innervating
B. sympathetic nerves
C. parasympathetic nerves
D. none of the above This answer is INCORRECT.
E. all of the above
Select the best answer: Pain impulses arising within the abdominal and thoracic cavities may reach the CNS by:
A. somatic nerves innervating
B. sympathetic nerves
C. parasympathetic nerves
D. none of the above
E. all of the above This answer is CORRECT!
All of the above are correct, since visceral pain is carried by them all.
- Question 6
- A
- B
At the level of the ventral trigeminothalamic tract, pain fibers are generally crossed or uncrossed?
A. Crossed
B. Uncrossed
At the level of the ventral trigeminothalamic tract, pain fibers are generally crossed or uncrossed?
A. Crossed This answer is CORRECT!
At the ventral trigeminothalamic tract, the fibers are already crossed and ascend to the thalamus.
B. Uncrossed
At the level of the ventral trigeminothalamic tract, pain fibers are generally crossed or uncrossed?
A. Crossed
B. Uncrossed This answer is INCORRECT.
- Question 7
- A
- B
- C
- D
- E
Cell bodies of first order pelvic visceral pain fibers are found in:
A. dorsal root ganglion
B. mesentric ganglion
C. superior cervical ganglia
D. inferior cervical ganglion
E. middle cervical ganglion
Cell bodies of first order pelvic visceral pain fibers are found in:
A. dorsal root ganglion This answer is CORRECT!
The cell bodies of the entire somatosensory system are located in the dorsal root ganglion.
B. mesentric ganglion
C. superior cervical ganglia
D. inferior cervical ganglion
E. middle cervical ganglion
Cell bodies of first order pelvic visceral pain fibers are found in:
A. dorsal root ganglion
B. mesentric ganglion This answer is INCORRECT.
C. superior cervical ganglia
D. inferior cervical ganglion
E. middle cervical ganglion
Cell bodies of first order pelvic visceral pain fibers are found in:
A. dorsal root ganglion
B. mesentric ganglion
C. superior cervical ganglia This answer is INCORRECT.
D. inferior cervical ganglion
E. middle cervical ganglion
Cell bodies of first order pelvic visceral pain fibers are found in:
A. dorsal root ganglion
B. mesentric ganglion
C. superior cervical ganglia
D. inferior cervical ganglion This answer is INCORRECT.
E. middle cervical ganglion
Cell bodies of first order pelvic visceral pain fibers are found in:
A. dorsal root ganglion
B. mesentric ganglion
C. superior cervical ganglia
D. inferior cervical ganglion
E. middle cervical ganglion This answer is INCORRECT.
- Question 8
- A
- B
- C
- D
- E
The following pathway is sectioned in a chordotomy for the treatment of pain:
A. Lateral spinothalamic tract
B. Ipsilateral dorsal column
C. Corticospinal tract
D. Spinocerebellar pathway
E. Spino-olivary tract
The following pathway is sectioned in a chordotomy for the treatment of pain:
A. Lateral spinothalamic tract This answer is CORRECT!
From the above nerve fibers, only the lateral spinothalamic tract carries pain sensation, and sections of these fibers will prevent pain information from getting to the brain.
B. Ipsilateral dorsal column
C. Corticospinal tract
D. Spinocerebellar pathway
E. Spino-olivary tract
The following pathway is sectioned in a chordotomy for the treatment of pain:
A. Lateral spinothalamic tract
B. Ipsilateral dorsal column This answer is INCORRECT.
C. Corticospinal tract
D. Spinocerebellar pathway
E. Spino-olivary tract
The following pathway is sectioned in a chordotomy for the treatment of pain:
A. Lateral spinothalamic tract
B. Ipsilateral dorsal column
C. Corticospinal tract This answer is INCORRECT.
D. Spinocerebellar pathway
E. Spino-olivary tract
The following pathway is sectioned in a chordotomy for the treatment of pain:
A. Lateral spinothalamic tract
B. Ipsilateral dorsal column
C. Corticospinal tract
D. Spinocerebellar pathway This answer is INCORRECT.
E. Spino-olivary tract
The following pathway is sectioned in a chordotomy for the treatment of pain:
A. Lateral spinothalamic tract
B. Ipsilateral dorsal column
C. Corticospinal tract
D. Spinocerebellar pathway
E. Spino-olivary tract This answer is INCORRECT.

