|
Figure 4.1 (see enlarged view) |
The features of the synaptic junction at the neuromuscular junction are shown in the figure at left. Skeletal muscle fibers are innervated by motor neurons whose cell bodies are located in the ventral horn of the spinal cord. The terminal region of the axon gives rise to very fine processes that run along skeletal muscle cells. Along these processes are specialized structures known as synapses. The particular synapse made between a spinal motor neuron and skeletal muscle cell is called the motor endplate because of its specific structure.
The synapse at the neuromuscular junction has three characteristic features of chemical synapses in the nervous system. First, there is a distinct separation between the presynaptic and the postsynaptic membrane. The space between the two is known as the synaptic cleft. The space tells us there must be some intermediary signaling mechanism between the presynaptic neuron and the postsynaptic neuron in order to have information flow across the synaptic cleft. Second, there is a characteristic high density of small spherical vesicles. These synaptic vesicles contain neurotransmitter substances. Synapses are also associated with a high density of mitochondria. Third, in most cases, there is a characteristic thickening of the postsynaptic membrane, which is due at least in part to the fact that the postsynaptic membrane has a high density of specialized receptors that bind the chemical transmitter substances released from the presynaptic neuron. Additional details on the morphological features of synaptic junctions is provided in Chapter 8 and Chapter 10.
4.2 Physiology of Synaptic Transmission at the Neuromuscular Junction
|
Figure 4.2 |
The figure at the right illustrates in a very schematic way how it is possible to study the physiology of synaptic transmission at the skeletal neuromuscular junction in great detail. A piece of muscle and its attached nerve are placed in a small experimental chamber filled with an appropriate Ringer solution. The resting potential of the muscle cell is recorded with a microelectrode. Electrodes are also placed on the surface of the nerve axon. Brief electric shocks cause action potentials to be initiated, which propagate to the synaptic terminal.
The figure below illustrates two types of potential changes that were recorded in such an isolated nerve-muscle preparation. The experiment also illustrates the properties of a powerful drug, curare, which has proven to be very useful in studying the process of synaptic transmission at the skeletal neuromuscular junction. Part A illustrates the sequence of potential changes recorded in the muscle cell as a result of stimulating the motor axon. The arrow indicates the point in time when the shock is delivered to the motor axon. Note that there is a quiescent period of time after the shock. The delay is due to the time it takes for the action potential in the motor axon to propagate from its site of initiation. After the delay, there are two types of potentials recorded in the muscle cell. First, there is a relatively slowly changing potential that will be the focus of the following discussion. If that slow initial potential is sufficiently large, as it normally is in skeletal muscle cells, a second potential, an action potential, is elicited in the muscle cell.
|
Figure 4.3 |
Action potentials in skeletal muscle cells are due to ionic mechanisms similar to those discussed previously. Specifically, there is a voltage-dependent change in Na+ permeability followed by a delayed increase in K+ permeability. (For smooth muscle cells and cardiac muscle cells the ionic mechanisms are different, however.)
The underlying event that triggers the action potential can be revealed by taking advantage of curare, an arrow poison used by some South American Indians. A low dose of curare (Part B) reduces the underlying event, but it is still not sufficiently reduced to fall below threshold. If a somewhat higher dose of curare is delivered (Part C), the slow underlying event becomes subthreshold. The underlying signal is known as the endplate potential (EPP) because it is a potential change recorded at the motor endplate. Generally, it is known as an excitatory postsynaptic potential (EPSP).
Curare blocks the endplate potential because it is a competitive inhibitor of acetylcholine (ACh), the transmitter released at the presynaptic terminal. Curare does not block the voltage-dependent Na+ conductance or the voltage-dependent K+ conductance that underlies the muscle action potential. Curare affects the stimulus (the EPSP) which normally leads to the initiation of the muscle action potential. An animal that is poisoned with curare will asphyxiate because the process of neuromuscular transmission at respiratory muscles is blocked.
Normally, the magnitude of the endplate potential is quite large. Indeed, the amplitude of the endplate potential is about 50 mV, but only about 30 mV is needed to reach threshold. The extra 20 mV is called the safety factor. Therefore, even if the endplate potential were to become somewhat smaller (e.g., 40 mV in amplitude) because of fatigue, the EPP would reach threshold and the one-to-one relationship between an action potential in the motor axon and an action potential in the muscle cell would be preserved.
|
Figure 4.4 |
4.3 Propagation of the EPP
What are the properties of the EPP and how does it compare with the properties of the action potential?
Is the endplate potential due to a voltage-dependent change in Na+ and K+ permeabilities like the action potential?
Is the endplate potential propagated in an all-or-nothing fashion like the action potential?
The figure on the left illustrates an experiment that examines the propagation of the endplate potential. The muscle fiber is impaled repeatedly with electrodes at 1 mm intervals. (Note that the endplate potential is small because this experiment is done in the presence of a low concentration of curare so the endplate potential can be recorded without the complications of triggering an action potential.) The endplate potential is not propagated in an all-or-nothing fashion. It does spread along the muscle, but it does so with decrement. Thus, the spread of the endplate potential from its site of initiation to other sites along the muscle cell occurs passively and with decrement, just as a subthreshold potential change in one portion of the axon spreads along the axon, or just as a change in temperature at one point on a metal rod spreads along the rod.
4.4 Overview of the Sequence of Events Underlying the EPP
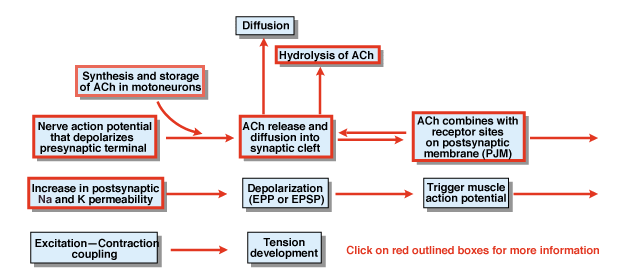
|
Figure 4.5 |
What are the other steps in the process of chemical synaptic transmission? Figure 4.5 provides an overview. A nerve action potential that is initiated in the cell body of a spinal motor neuron propagates out the ventral roots and eventually invades the synaptic terminals of the motor neurons. As a result of the action potential, the chemical transmitter acetylcholine (ACh) is released into the synaptic cleft. ACh diffuses across the synaptic cleft and binds to special receptors on the postsynaptic or the postjunctional membrane. The binding of ACh to its receptors produces a conformational change in a membrane channel that is specifically permeable to both Na+ and K+. As a result of an increase in Na+ and K+ permeability, there is a depolarization of the postsynaptic membrane. That depolarization is called the endplate potential or more generally the EPSP. If the EPSP is sufficiently large, as it normally is at the neuromuscular junction, it leads to initiation of an action potential in the muscle cell. The action potential initiates the process of excitation contraction coupling and the development of tension. The duration of the endplate potential is about 10 msec.
Two factors control the duration of the EPSP at the neuromuscular junction. First, ACh is removed by diffusion. Second, a substance in the synaptic cleft, called acetylcholinesterase (AChE), hydrolyzes or breaks down ACh. AChE is one of the most efficient enzymes known. A single molecule of AChE can hydrolyze 600,000 molecules of ACh per minute.
4.5 Role of AChE
Figure 4.6 |
An important family of substances, one of which is neostigmine, inhibits the action of AChE. Neostigmine blocks the action of AChE, and thereby makes the endplate potential larger and longer in duration. This figure illustrates two endplate potentials. One was recorded in saline and curare and a second recorded after neostigmine was added to the solution. (Curare is added so that the properties of the EPP can be studied without triggering an action potential in the muscle cell.) After applying neostigmine the endplate potential is much larger and longer in duration.
4.6a Myasthenia Gravis
Myasthenia gravis is associated with severe muscular weakness because of a decrease in the number of acetylcholine receptors in the muscle cell. If the endplate potential is smaller, the endplate potential will fail to reach threshold. If it fails to reach threshold, there will be no action potential in the muscle cell and no contraction of the muscle, which causes muscular weakness. Neostigmine and other inhibitors of AChE are used to treat patients with myasthenia gravis. These agents make the amount of acetylcholine that is released more effectively reach the remaining acetylcholine receptors.
4.6b Nerve Agents
Although inhibitors of AChE have important therapeutic value, some inhibitors have been, and are still used as poisons. Some AChE inhibitors such as Soman and Sarin form a fairly irreversible block of AChE. This block leads to extreme levels of ACh in the synaptic cleft. Individuals so poisoned die from seizures and muscle spasticity including respiratory muscles.
Figure 4.7 |
4.7 Iontophoresis of ACh
Iontophoresis is an interesting technique that can be used to further test the hypothesis that ACh is the neurotransmitter substance at the neuromuscular junction. If ACh is the transmitter that is released by this synapse, one would predict that it should be possible to substitute artificial application of the transmitter for the normal release of the transmitter. Since ACh is a positively charged molecule, it can be forced out of a microelectrode to simulate the release of ACh from a presynaptic terminal.
Figure 4.8 |
Indeed, minute amounts of ACh can be applied to the vicinity of the neuromuscular junction. Figure 4.8 compares an EPP produced by stimulation of the motor axon and the response to ejections of ACh. The potential change looks nearly identical to the endplate potential produced by the normal release of ACh. This experiment provides experimental support for the concept that ACh is the natural transmitter at this synapse.
The response to the ejection of ACh has some other interesting properties that are all consistent with the cholinergic nature of the synapse at the skeletal neuromuscular junction. Neostigmine makes the response to the iontophoresis of ACh longer and larger. Curare reduces the response because it competes with the normal binding of ACh. If ACh is ejected into the muscle cell, nothing happens because the receptors for acetylcholine are not in the inside; they are on the outside of the muscle cell. Application of acetylcholine to regions of the muscle away from the end-plate produces no response because the receptors for the ACh are concentrated at the synaptic region.
To test your understanding so far, consider how an agent such as TTX would affect the generation of both an EPP and the response of a muscle fiber to the iontophoretic application of ACh? TTX has no effect on the response to ACh, but it does block the EPP. The reason the response to ACh is unaffected is clear, but many expect that if there is no effect here, there should be no effect on the EPP either. Tetrodotoxin does not affect the binding of acetylcholine to the receptors and therefore will not affect the response to direct application of ACh. However, tetrodotoxin will affect the ability of an action potential to be elicited in the motor axon. If an action potential cannot be elicited in the motor axon, it cannot cause the release of transmitter. Thus, tetrodotoxin would totally abolish the EPP. The block would not be due to a block of ACh receptors, but rather to a block of some step prior to the release of the transmitter.
4.8 Ionic Mechanisms of the EPP
Bernard Katz and his colleagues were pioneers in investigating mechanisms of synaptic transmission at the neuromuscular junction. They suggested that the channel opened by ACh was one that had equal permeability to both Na+ and K+. Because it was equally permeable to Na+ and K+, Katz suggested that, as a result of the opening of these channels, the membrane potential would move toward 0 mV. (A value of alpha in the GHK equation equal to one, which when substituted into the equation, yields a potential of about 0 mV.)
Figure 4.9 |
The experiment shown in the figure on the left tests that concept. The muscle cell has been penetrated with a recording electrode as well as another electrode that can be connected to a suitable source of potential in order to artificially change the membrane potential. Normally, the membrane potential is about -80 mV [Skeletal muscle cells have higher (more negative) resting potentials than most nerve cells.] Again, a small amount of curare is added so that the EPP is small. Katz noticed in these experiments that the size of the EPP changed dramatically depending upon the potential of the muscle cell. If the membrane potential is moved to 0 mV, no potential change is recorded whatsoever. If the membrane potential is made +30 mV, the EPP is inverted. So three different stimuli produce endplate potentials that are very different from each other.
The lack of a response when the potential is at 0 mV is particularly informative. Consider why no potential change is recorded. Presumably, the transmitter is being released and binding to the receptors. The simple explanation for a lack of potential change is that the potential at which the opening of ACh channels are trying to reach has already been achieved. If the membrane potential is made more positive than 0 mV, then the EPP is inverted. No matter what the potential, the change in permeability tends to move the membrane potential towards 0 mV! If the resting potential is more negative than 0 mV, there is an upward deflection. If it is more positive, there is a downward deflection. If it is already at 0 mV, there is no deflection.
Figure 4.10 |
This potential is also called the reversal potential, because it is the potential at which the sign of the synaptic potential reverses. The experiment indicates that, as a result of ACh binding to receptors, specific channels become equally permeable to Na+ and K+. This permeability change tends to move the membrane potential from wherever it is initially towards a new potential of 0 mV.
Why does the normal endplate potential never actually reach 0 mV? One reason is that the sequence of permeability changes that underlie the action potential "swamp out" the changes produced by the EPP. But even if an action potential was not triggered, the EPP still would not reach 0 mV. This is because the ACh channels are only a small fraction of the total number of channels in muscle fibers. The K+ channels that endow a muscle cell with its resting potential are present as well. Their job is to try to maintain the cell at the resting potential.
The channel opened by ACh is a member of a general class of channels called ligand-gated channels or ionotropic receptors. As illustrated in Figure 4.10, the transmitter binding site is part of the channel itself. As a result of transmitter binding to the receptor (generally two molecules are necessary), there is a conformational change in the protein allowing a pore region to open and ions to flow down their electrochemical gradients. Additional details of the channel are presented in Chapter 11.
Test Your Knowledge
An endplate potential in a skeletal muscle cell could in principle be produced by a decreased permeability to which of the following ions(s)? (Assume that there is a finite initial permeability to each of the ions listed below and that physiological concentration gradients are present.): A. Na+ B. Na+ and Ca2+ C. Ca2+ D. K+ An endplate potential in a skeletal muscle cell could in principle be produced by a decreased permeability to which of the following ions(s)? (Assume that there is a finite initial permeability to each of the ions listed below and that physiological concentration gradients are present.): A. Na+ This answer is INCORRECT. B. Na+ and Ca2+ C. Ca2+ D. K+ An endplate potential in a skeletal muscle cell could in principle be produced by a decreased permeability to which of the following ions(s)? (Assume that there is a finite initial permeability to each of the ions listed below and that physiological concentration gradients are present.): A. Na+ B. Na+ and Ca2+ This answer is INCORRECT. C. Ca2+ D. K+ An endplate potential in a skeletal muscle cell could in principle be produced by a decreased permeability to which of the following ions(s)? (Assume that there is a finite initial permeability to each of the ions listed below and that physiological concentration gradients are present.): A. Na+ B. Na+ and Ca2+ C. Ca2+ This answer is INCORRECT. D. K+ An endplate potential in a skeletal muscle cell could in principle be produced by a decreased permeability to which of the following ions(s)? (Assume that there is a finite initial permeability to each of the ions listed below and that physiological concentration gradients are present.): A. Na+ B. Na+ and Ca2+ C. Ca2+ D. K+ This answer is CORRECT! |






